
Research Education
CLINICAL TRIALS are conducted in phases.
Each phase has a different purpose
Each clinical trial has different eligibility criteria that determine whether a person can participate. The criteria are used to help researchers answer the study questions and to ensure the safety of all volunteers. Examples of eligibility criteria include the type and stage of disease, health history, and past or current treatment.
If you are eligible for a clinical trial, you will be given information that will help you decide whether or not to participate.
Why does Diversity matter in clinical trials, and how does QCR strive to improve diversity?
Is Research for me?
It’s true that clinical research studies are open to everyone, and participation can offer benefits such as access to potentially life-changing treatments, medical monitoring and care, and the opportunity to contribute to medical knowledge and the development of new treatments.
However, it’s important to understand that clinical research studies may not be suitable for everyone. Some studies have strict eligibility criteria based on factors such as age, medical history, and current health status. It’s also important to carefully weigh the potential benefits of participation against the risks and potential side effects of the treatment being studied.
Before deciding to participate in a clinical research study, it’s important to discuss your options with your healthcare provider and the study team, carefully review the study information and consent documents, and ask any questions you may have. You should also consider the potential impact of participation on your personal and professional life, as well as any travel or other logistical requirements.
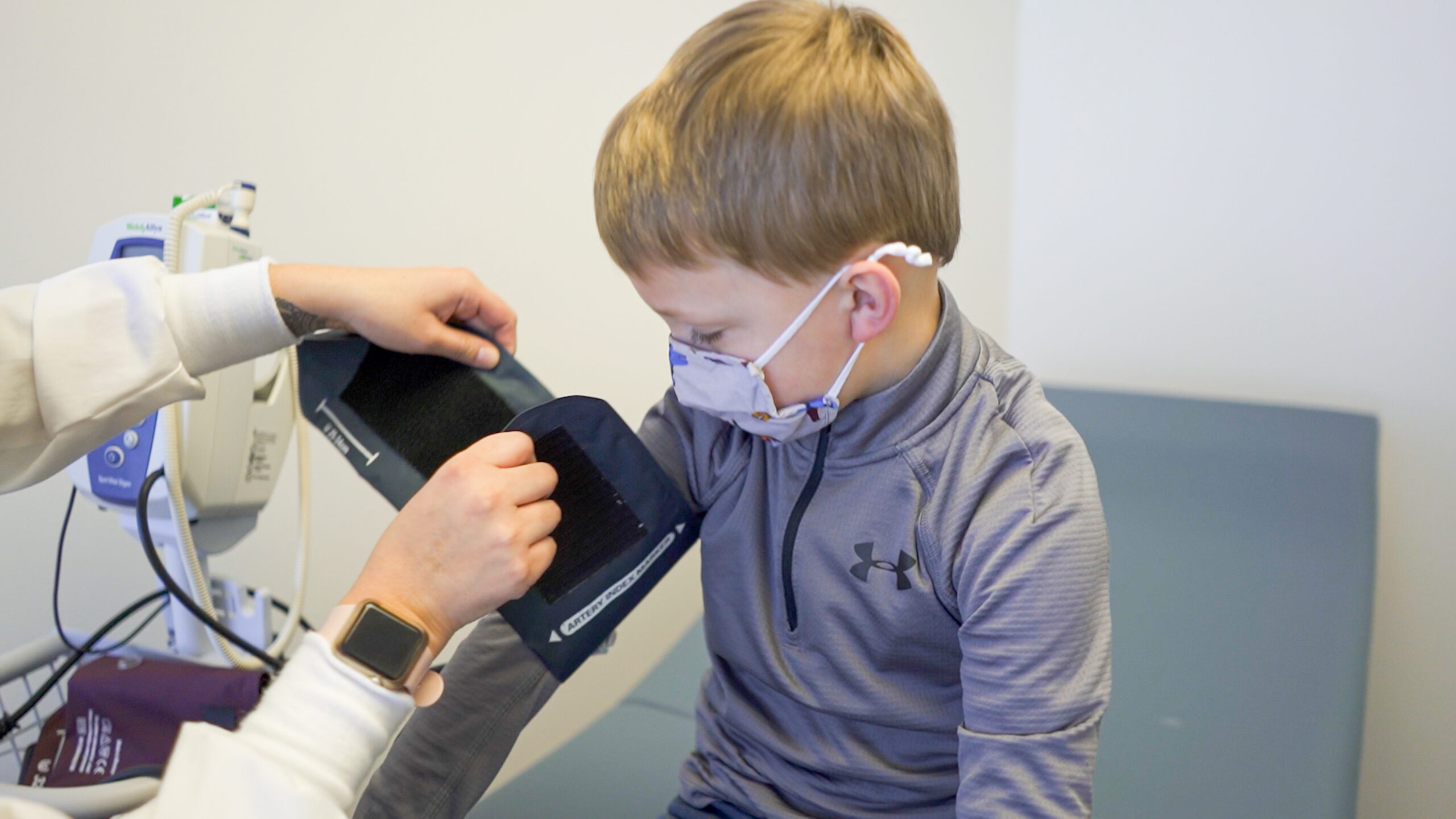
Community Impact
Clinical trials can have a significant impact on the community in various ways
Here are some ways clinical trials can impact the community:

Community Impact
Clinical trials can have a significant impact on the community in various ways

Here are some ways clinical trials can impact the community:

